Contact: Craig Blanchette
We have also been developing biophysical methods to measure mechanical and structural changes in cells under physiological relevant conditions. These methods primarily utilize combined atomic force microscopy (AFM) and confocal fluorescent microscopy (CFM). This work has focused primarily on two projects: 1) measuring changes to cytoskeletal elements in response to localized delivery of pathogenic stimuli (Internalin A (InlA) from Listeria monocytogenes) and 2) measuring the mechanical stiffness while imaging changes to the cytoskeletal elements of chondrocytes in the presence and absence of cytoskeletal disruptors.
Localized delivery of pathogenic stimuli to cells:
In the studies involving localized delivery of InlA, AFM tips containing beads that had been coated with InlA (pathogen mimetic) were brought into contact with Madin Darby Canine Kidney (MDCK) cells that had been transfected with Actin-GFP. Upon contact, the cells were imaged by CFM, which allowed us to visually observed rearrangement of cytoskeletal elements upon localized stimulation with the pathogenic mimietic. In these experiments, we observed actin filaments coalesce and form a ring that wrapped around the InlA coated beads (Figure 2 and movie 1). During this process we observed a force being applied to the AFM cantilever, indicating that the cell was attempting to pull the bead in through phagocytosis. The methods developed in these studies will prove valuable in understanding the cytoskeletal and mechanical response to localized delivery of a pathogen mimetic and can be used to study the interaction between different pathogen mimetics and a variety of different cell types.
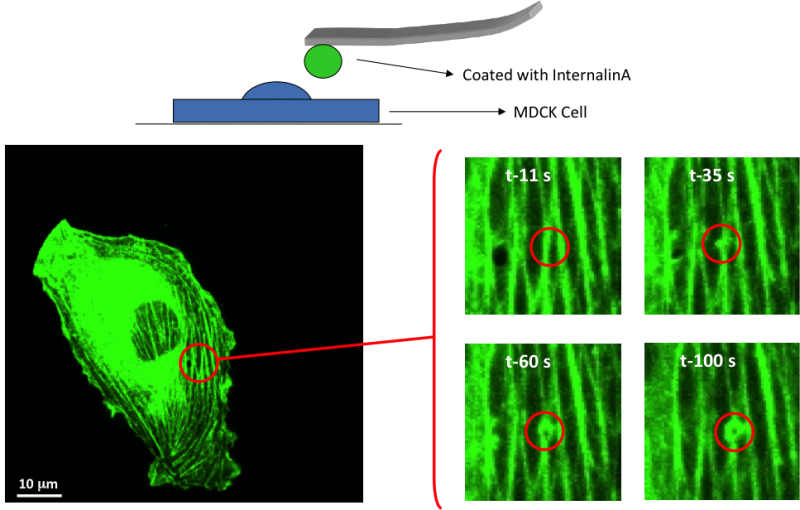
Video: Probing and individual cell with a pathogenic mimetic and measure the cytoskeletal response.
Simultaneously measuring the mechanical stiffness and cytoskeletal response in chondrocytes subjected to a load in the presence and absence of cytoskeletal disruptors
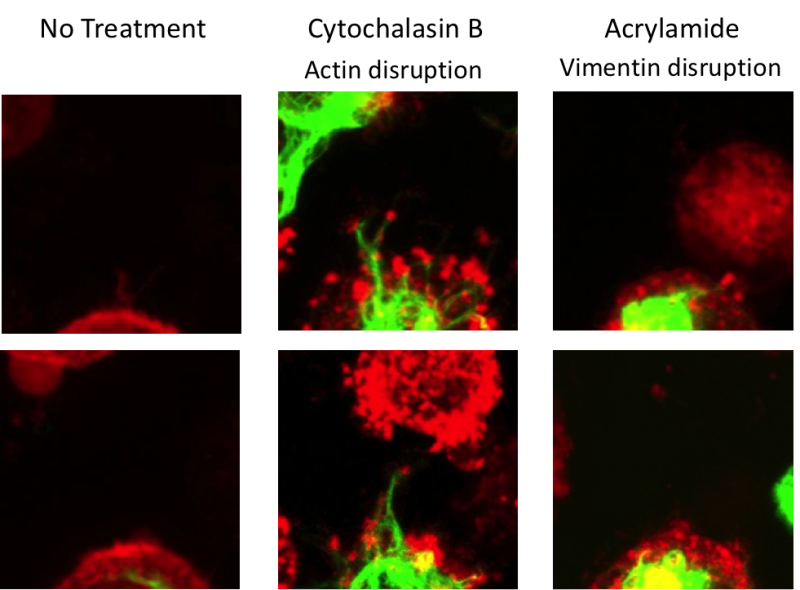
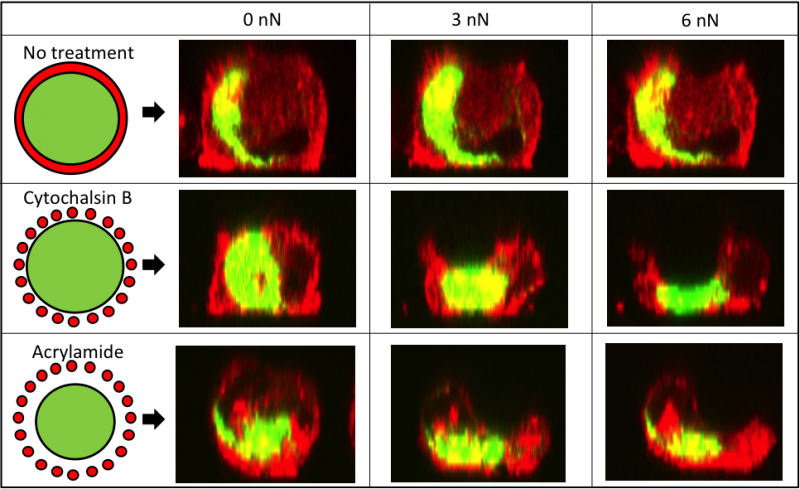
In this project, we systematically evaluated the effect of cytoskeletal disruptors on the mechanical properties and cytoskeletal response of chondrocytes as a function of deformation. By using an integrated AFM and confocal fluorescent microscope, we were able to monitor cytoskeletal and biomechanical deformation in transgenic cells (GFP-vimentin and mCherry-actin) under compression. For these experiments, the chondrocytes were mechanically compressed by a 10 µm bead attached to the AFM cantilever. We found that the elastic modulus of chondrocytes in the absence of cytoskeletal disruptors decreased with increased indentation (15-2000 nm). However, when the actin or the intermediate filament structures were chemically disrupted, the mechanical properties of chondrocytes were altered and there was a decrease in both the elastic modulus and viscoelastic properties, resulting in a dramatic loss of the indentation-dependent response. Actin and vimentin cytoskeletal structures were monitored using confocal fluorescent microscopy in transgenic cells treated with disruptors, and both treatments had a profound disruptive effect on the actin filaments (Figure 3). Here we show that disrupting the structure of intermediate filaments indirectly altered the configuration of the actin cytoskeleton, which allowed for rapid deformation of the cells under a small load (Figure 4). These findings underscore the importance of the cytoskeletal elements in the overall mechanical response of chondrocytes, indicating that intermediate filament integrity is key to the non-linear elastic properties of chondrocytes. These studies helped to improve our understanding of the mechanical properties of articular cartilage at the single cell level.
____________________________________
